Generating electricity outside cities and transporting it into the city comes with a lot of power loss. Ideally, as much of it as possible should be generated locally. Scientists have found a way to alleviate this issue by developing a new type of transparent solar cell that can be used in the windows of buildings and is expected to last for 30 years.
Tall buildings have a lot of solar energy potential with loads of glass surface - Song_about_summer via Shutterstock / HDR tune by Universal-Sci
The need for eco-friendly energy production is increasing at a rapid pace. According to research, solar is one of the cheapest methods to generate electricity. A particular problem with solar power stations is that they take up a lot of space, and while cities generally need the most electricity, they have the least amount of space for power plants.
Transporting electricity generated elsewhere into cities through power lines results in energy loss, so, as mentioned above, it would be more efficient to generate as much electricity as possible inside cities.
While silicon is currently still the most efficient material for solar panels, it is not transparent. Researchers at the University of Michigan, North Carolina State University, Tianjin University, and Zhejiang University have therefore been looking into organic or carbon-based materials for window-friendly solar panels. They published their work in the science journal: Nature Communications
The main challenge was figuring out how to keep highly efficient organic light-converting materials from rapidly deteriorating when used. The resilience of these materials is derived from the molecules that move photogenerated electrons to the electrodes. These materials are called "non-fullerene acceptors" to distinguish them from the more sturdy but less efficient "fullerene acceptors" created from nanoscale carbon mesh. Solar cells made with non-fullerene acceptors that incorporate sulfur can reach efficiencies of 18% (an efficiency level that rivals silicon), but the problem is that they have a very short lifespan.
Yongxi Li, first author of the study, explains that non-fullerene acceptors cause very high efficiency but incorporate weak bonds that easily separate under high-energy photons, especially with ultraviolet photons found in sunlight.
After examining the nature of the non-fullerene acceptor deterioration, the researchers discovered that the exposed solar cells only needed reinforcements in a few spots. Firstly, ultraviolet rays would have to be blocked off. To do so, the team added a coating of zinc oxide, a popular sunscreen component on the side of the glass that faces the sun.
A thinner zinc oxide layer next to the light-absorbing area improves the conduct of solar-generated electrons to the electrode. Sadly, this also tears down the fragile light absorber. The researchers added a layer of a carbon-based material called IC-SAM as a buffer to solve this issue.
On top of that, the electrode that draws positively charged "holes" into the circuit (essentially spaces abandoned by electrons) can react with the light absorber. So to protect that flank, the team added another buffer layer in the form of a fullerene shaped like a soccer ball. (A fullerene is an allotrope of carbon whose molecule consists of carbon atoms joined by single and double bonds to form a closed mesh, with fused rings of five to seven atoms.)
The researchers then put their new reinforcements to the test under various intensities of simulated sunshine, ranging from 1 sun to 27 suns, and temperatures as high as 65 degrees celsius. They projected that the solar cells would still be functioning at 80% efficiency after 30 years based on how performance declined under these conditions.
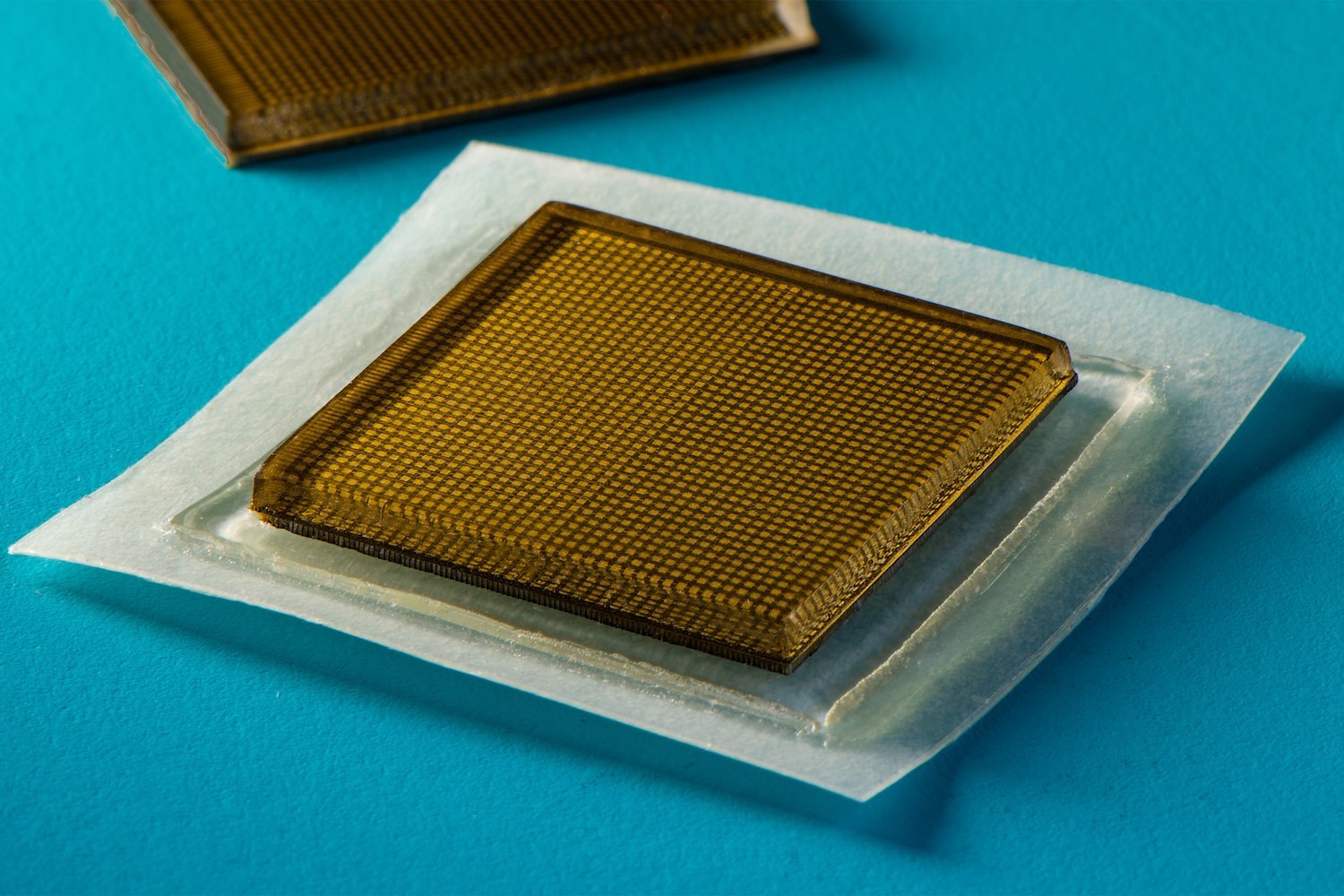
One of the researchers holding a solar cell module with 40% transparency, based on the new design with an estimated life expectancy of 30 years - (Image Credit: Robert Coelius, University of Michigan Engineering)
After examining the nature of the non-fullerene acceptor deterioration, the researchers discovered that the exposed solar cells only needed reinforcements in a few spots. Firstly, ultraviolet rays would have to be blocked off. To do so, the team added a coating of zinc oxide, a popular sunscreen component on the side of the glass that faces the sun.
A thinner zinc oxide layer next to the light-absorbing area improves the conduct of solar-generated electrons to the electrode. Sadly, this also tears down the fragile light absorber. The researchers added a layer of a carbon-based material called IC-SAM as a buffer to solve this issue.
On top of that, the electrode that draws positively charged "holes" into the circuit (essentially spaces abandoned by electrons) can react with the light absorber. So to protect that flank, the team added another buffer layer in the form of a fullerene shaped like a soccer ball. (A fullerene is an allotrope of carbon whose molecule consists of carbon atoms joined by single and double bonds to form a closed mesh, with fused rings of five to seven atoms.)
The researchers then put their new reinforcements to the test under various intensities of simulated sunshine, ranging from 1 sun to 27 suns, and temperatures as high as 65 degrees celsius. They projected that the solar cells would still be functioning at 80% efficiency after 30 years based on how performance declined under these conditions.
An extreme close-up of a cross-sectional slice of an OPV with the added layers of material (IC-SAM and C70) between the organic material and the external buffers. After the material was subjected to high-intensity light to replicate an estimated age of 30 years. It reveals an intact organic active region with no breakdown at the edges. (Image Credit: Kan Ding, University of Michigan)
Because the materials can be prepared as liquids, the manufacturing costs are expected to be relatively low, increasing the feasibility for wide-scale use. All in all, we are yet another step closer to actual solar power-generating windows with these findings.
Further reading:
Extraordinary new material will enable us to store solar energy for an extended period of time (Universal-Sci)
Should we turn the Sahara Desert into a giant solar farm? (Universal-Sci)
The full cost of electricity (University of Texas at Austin white paper)
Non-fullerene acceptor organic photovoltaics with intrinsic operational lifetimes over 30 years (Nature Communications)
If you enjoy our selection of content, consider subscribing to our newsletter
FEATURED ARTICLES: